Improving Automatic Dish Washing Detergency with the Optimal Green Chelate
Published
November 18, 2021
In 2013, New York and Maryland enacted legislation that prohibited the sale of commercial dishwasher detergents that contain more than a trace quantity of phosphorus – defined as 0.5%. This ban followed the 2010 ban on household use of phosphate-containing dishwasher detergents that was enacted by 17 states in the United States. The industry is still working to replace phosphates with solutions that clean as well, especially in regions with hard water.
Role of Phosphates in Automatic Dishwashing
Phosphates provide and control alkalinity, ensuring that a detergent has the proper pH to remove fatty soils and prevent them from depositing on surfaces. As a builder, a phosphate will also strengthen the action of other ingredients. They soften water by sequestering and precipitating calcium and magnesium ions. Phosphates also break up large soil particles and prevent small soil particles from combining. But they are not the only chemicals that can do this: the chelates Ethylenediaminetetraacetic acid (EDTA), Nitrilotriacetic acid (NTA), and polycarboxylates play similar roles in automatic dishwashing.
Improving Phosphate-free Formulas with Green Chelates
In addition to phosphates, chelating agents like EDTA, NTA and polycarboxylates are also under scrutiny for their slow biodegradability. For this reason, many formulators are turning to what we call “green chelates.” Green chelates are readily biodegradable materials used in automatic dishwashing to prevent scale formation, suppress filming, and boost the effectiveness of biocides. Green chelates are segmented into sodium gluconate (NaC6H11O7), ethylenediamine-N,-N'-disuccinic acid (EDDA), L-glutamic acid N-N-diacetic acid (GLDA), methyl glycindiacetic acid (MGDA), and others.
MGDA as an Alternative
MGDA, patented by BASF as Trilon® M, is a strong, readily biodegradable complexing agent used to enhance the cleaning efficiency of Institutional automatic dishwashing detergents. Trilon M’s properties make it an ideal substitute for phosphate. It demonstrates high performance, can be added to neutral, alkaline and acidic formulations, and it has superior ecological and toxicological profiles.Trilon M was the first product to meet the Environmental Protection Agency's (EPA) Design for the Environment (DfE) certification requirements for chelating and sequestering agents. Trilon M meets Safer Choice Standards and therefore is listed on CleanGredients®, the U.S. EPA’s definitive database of chemical ingredients whose formulations have been pre-approved.
Proof of Performance
When compared with sodium carbonate, sodium hydroxide (NaOH), and sodium triphosphate, Trilon M performed equally as well, or better than any alternative, including the phosphate and the leading commercial non-phosphate gel on the market.

When compared with other complexing agents, Trilon M demonstrates less spotting on silverware.

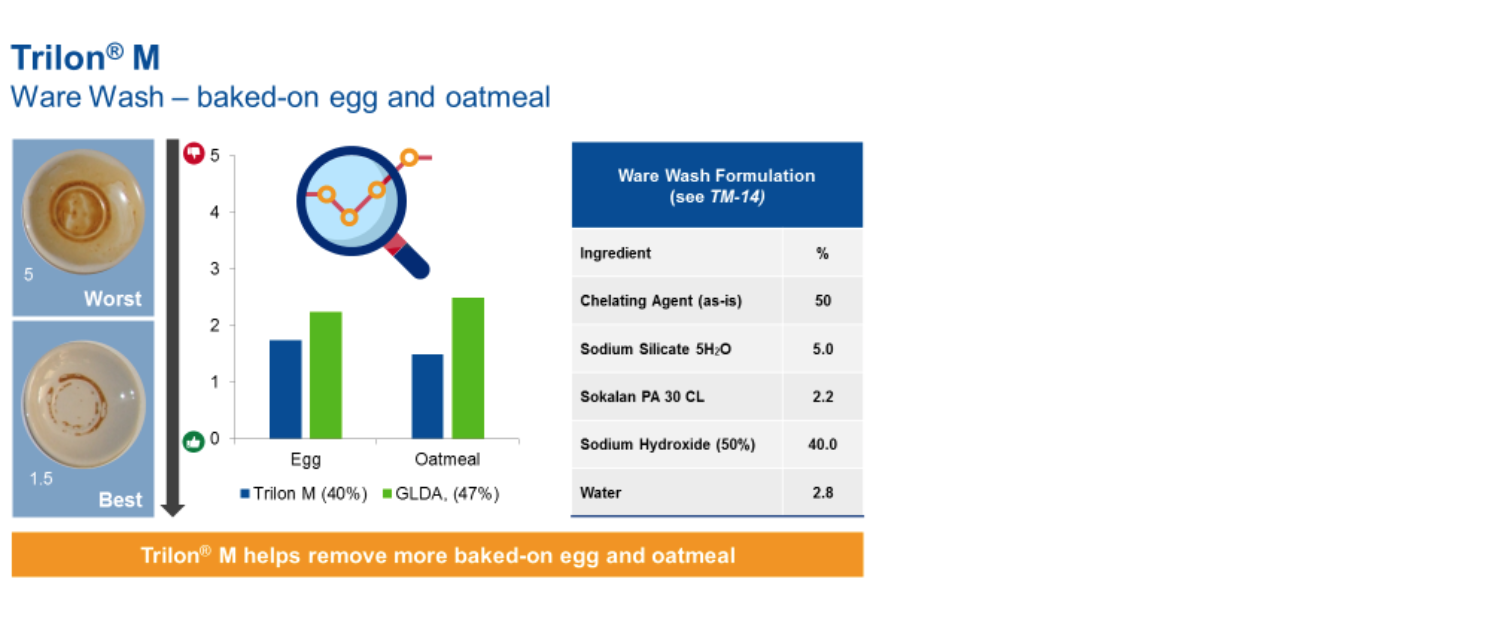
Strong chelates are required in I&I automatic dish washing due to the limited mechanical action in the cleaning process. When compared with N,N-Dicarboxymethyl glutamic acid tetrasodium salt (GLDA), Trilon M delivers superior performance on both protein and carbohydrate soils.
Trilon M is also more effective than sodium citrate in removing tough stains, like tea, that are affixed to hydrophilic dish surfaces by hard water cations.

Advancing Automatic Dishwashing Formulation with Trilon® M
By taking advantage of a performance synergy between Trilon® M and sodium citrate (in formulations that have little to no amounts of caustic), formulators can achieve strong performance at cost competitive prices.Consider the example with three different formulas: they have different chelating agents, yet are priced similarly. Formula 1 has the most chelating agent by weight in the formula, because it is only using the sodium citrate, a commodity.Formula 2 uses a combination of Trilon M and citrate, at the same cost as Formula 1, but with less total chelating agent (some of the citrate in Formula 1 has been replaced by Trilon M, and the rest with filler). Formula 3 uses only the powerful and more expensive Trilon M, but the difference in cost is off-set by a cheaper filler. Formula 2 achieves considerably better performance, reaching close to the premium performance level. This synergistic combination of Trilon M and citrate can be used to enhance formula performance without additional cost. By investing in more Trilon M, the formulator can achieve premium performance with absolutely no spotting or filming, as illustrated with Formula 4.

Conclusion
As formulators continue to advance the development of safe and environmentally-friendly options for Industrial and Institutional automatic dishwashing solutions, Trilon® M plays a critical role. Its performance is superior to phosphates, its sustainability profile is unmatched by any other chelates, and its formulation flexibility is unparalleled.


